With no vaccine anytime soon, how will we treat COVID-19?
The science behind stopping this spiky spherical virus
Expert opinion suggests that our most permanent paths out of the COVID-19 pandemic will be to develop either a vaccine or a treatment. Unfortunately, we're likely at least 12 months away from deploying a coronavirus vaccine. So what can we expect in terms of treatments?
The international scientific and medical communities are scrambling to answer that question. To do that, they have to learn from the successes and failures of treating the last two outbreaks of coronaviruses, and learn more about how the SARS-CoV-2 virus infects cells. By understanding how the virus works, researchers can pinpoint which processes are most vulnerable to be dismantled with drugs. Human trials are underway to test many existing drugs on people who need help right now, and labs across the globe are rushing to create and test others.
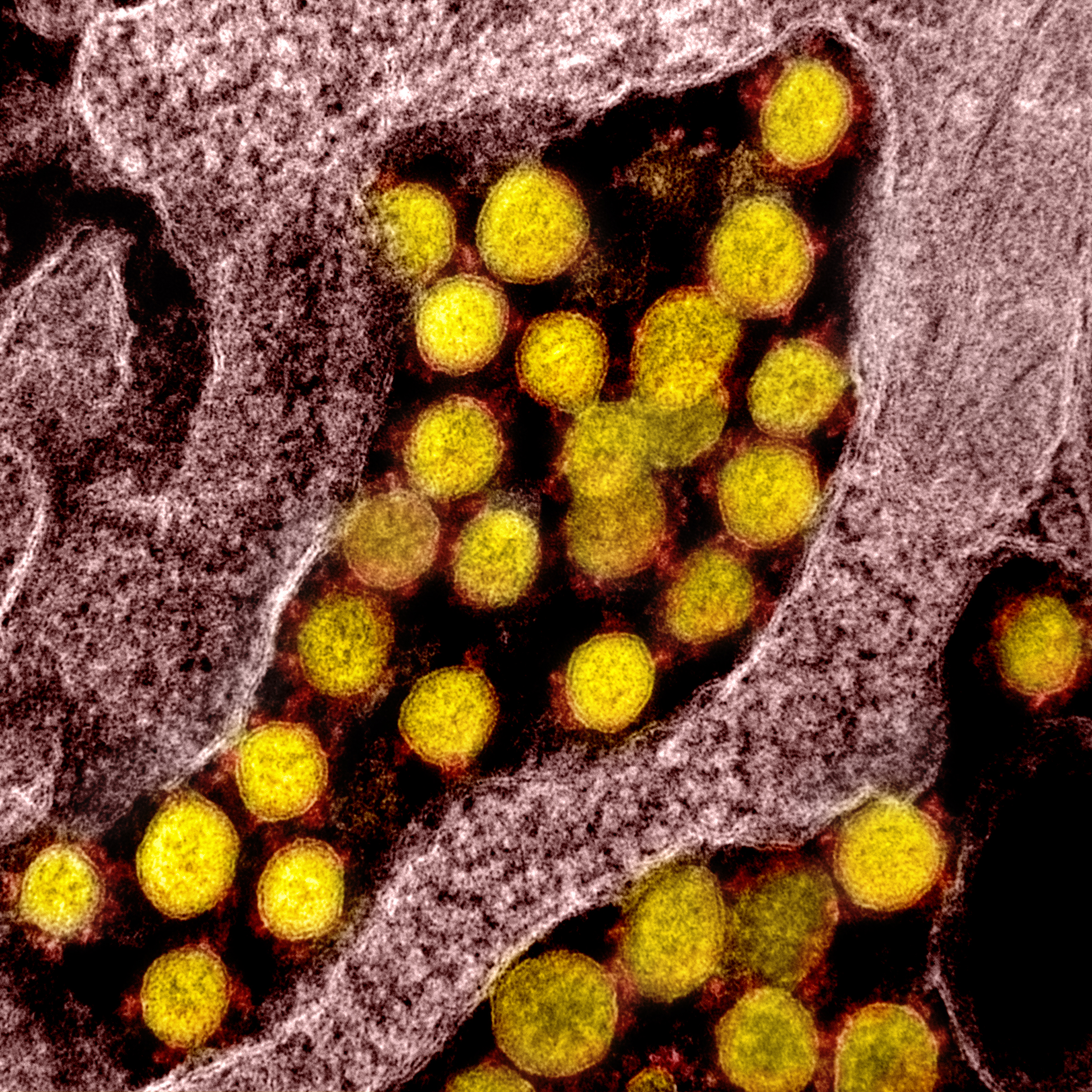
Coronavirus (SARS-CoV-2, in yellow) particles isolated from a patient
National Institute of Allergy and Infectious Diseases, NIH
To understand what all of these treatments are, it’s helpful to consider what they’re trying to treat. SARS-CoV-2, like its relative SARS, is covered in tiny protein “spikes,” or S-Proteins. Those spikes are the key to getting the virus to infiltrate our otherwise healthy cells. But, despite their name, the spike proteins don’t poke and slash a path into any human cell — the actual process is far more elegant.
It turns out that the S-Protein attaches really well to a different protein that dots the outside of some of our cells. That protein, the angiotensin converting enzyme 2, or ACE2, sits on the surface of some cells in our lungs, and other organs like the kidney and heart. So when people are exposed to the virus, either through contaminated coughs or face-touches, the virus can sneak into their airways and latch on to the ACE2 on healthy cells. The attached SARS-CoV-2 then gets absorbed into the cell and releases its cargo: a short fragment of genetic code.
The cell reads this genetic code, a blueprint for its own self-destruction, and builds all the bits and pieces for the coronavirus to copy itself. The infected cell basically turns into a coronavirus factory. This is how one virus particle turns into billions and makes you sick.
As daunting as this all may sound, knowing how this process works helps scientists to invent treatments to stop it. In some cases, they don’t even have to invent anything. The drugs in clinical trials right now were invented years ago for other diseases.
Human trials are underway to repurpose existing antiviral drugs. In the US, multiple hospitals are testing remdesivir, a drug originally designed for Ebola, and hoping for better luck treating COVID-19. Remdesivir was considered to be the most promising candidate by the World Health Organization back in January. Preliminary reports from China suggest that favipiravir, a new flu medicine, could also be effective. And others are reporting some success with hydroxychloroquine and chloroquine — a drug dating back the 1930s to treat infections caused by the malaria parasite – though those claims have been scrutinized by some experts, and new studies suggest that they are not effective. One man died after taking a chloroquine product meant for cleaning fishtanks.
Each of these drugs goes after the virus’ ability to copy itself, and each may stop the infection from progressing to the full symptoms of coronavirus disease. While that’s clearly a valuable strategy, it’s also known that antivirals don’t work as well if given later in a person's infection — the key is diagnosing and treating early.
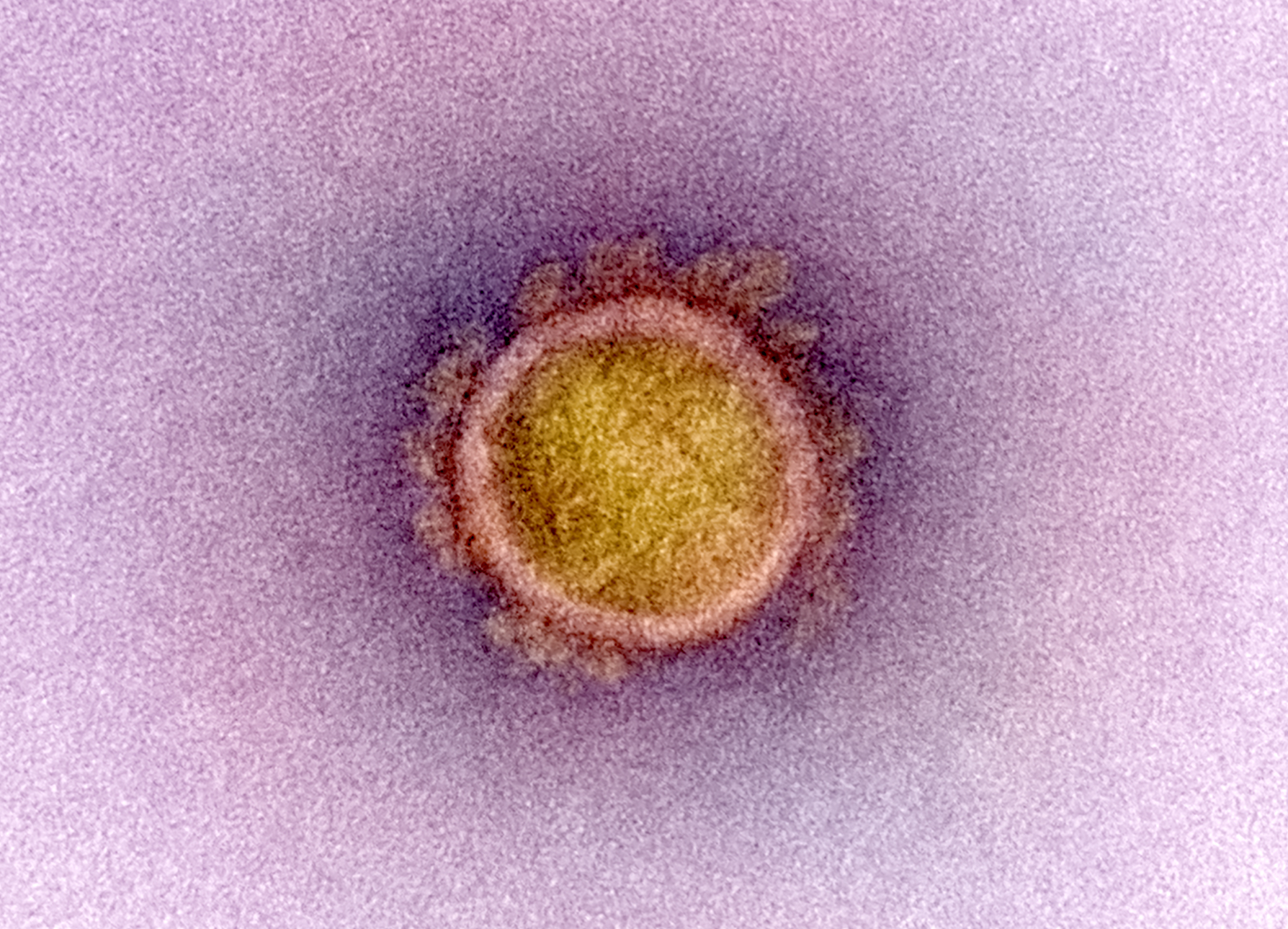
Microscope image of a single coronavirus particle showing the "spike proteins" on its round surface
National Institute of Allergy and Infectious Diseases, NIH
But a COVID-19 treatment doesn’t have to focus only on viral replication. We may also see successful drugs that go after completely different aspects of coronavirus infection. After the 2003 outbreak of SARS, researchers showed that cells react to coronavirus infections by reducing the amounts of ACE2 available for the virus to latch on to. That may sound like a good thing, but our bodies may rely on ACE2 to protect us from organ damage.
One proposed treatment currently in trials uses losartan, a blood pressure and diabetes medication. Losartan increases the levels of ACE2, the protein that the virus’ spikes target, and can prevent lung damage from COVID as a result. These ever-important ACE2 levels may even contribute to why the elderly are more susceptible to severe infections, as ACE2 levels appear to decrease with age.
Scientists are also proposing treatments that could prevent the virus from entering cells in the first place. One report suggests treating people with free-floating versions of ACE2. These proteins would serve as a sort of dummy target that intercepts SARS-CoV-2 before it can infect cells. (The authors declared a competing interest as the patent holders of a way to make soluble ACE2). Another recent study suggests that we could prevent coronavirus infection by deactivating a human protein that "softens" the spikes, making it easier for the virus to release its cargo.
Looming over this nowhere-near-exhaustive list of treatments is the fact that the world is squarely in the throes of a pandemic. There are hundreds of trials ongoing, but no treatments have yet been approved as safe and effective. In the absence of a safe, effective, and regulated treatment, extensive diagnostic testing is one of the best preventative measures that the world has.
South Korea has instituted widespread testing, even for people without symptoms. That approach has allowed the country to keep the virus in check without needing citywide lockdowns. The US is not even testing every person with symptoms. In a recent report, former Food and Drug Administration (FDA) commissioner Scott Gottlieb and Ian McClellan, director of the Duke-Margolis Center for Health Policy, called on the FDA to establish two task forces: one for rapidly advancing drug development, and the other for developing better, faster diagnostics.
In pursuit of better COVID-19 diagnostics, a team led by Florian Krammer of the Icahn School of Medicine recently developed a blood test to detect who has been infected, and which people have developed immunity. Krammer has suggested that we could use this information to better test and count people infected, and even treat people using blood from people with immunity. Scientists at Johns Hopkins University and elsewhere are actively working on this sort of “immune therapy” for COVID-19.

