Via Wikimedia
After decades of work, an effective cytomegalovirus vaccine is on the horizon
Monoclonal antibodies reveal key vaccine structure
Cytomegalovirus infection before birth is a leading cause of sickness affecting children's development.
There are no approved vaccines to prevent this infection, which happens when a pregnant person is exposed to cytomegalovirus (CMV) and the virus passes through the placenta to the fetus. To better evaluate vaccine candidates for clinical trials, we need an "immune correlate of protection," a sign that helps us predict whether a vaccine will protect against CMV infection and disease. Such a sign was discovered through a study conducted under the leadership of Sallie R. Permar, a physician-scientist at the Duke University School of Medicine.
CMV infection in healthy children and adults is common and usually asymptomatic or has mild, flu or cold-like symptoms. CMV awareness is fairly poor. In 2011, the US Congress even passed a resolution naming June National CMV Awareness Month. This recognition aims to increase awareness of CMV exposure risks. Young children are a common source of CMV. So, there may be a greater risk of congenital CMV infection for people who have frequent contact with young children. This awareness helps reduce the spread of CMV and makes early treatment possible for those severely affected.
The primary objective of this study was to define the immune responses elicited by a CMV vaccine tested in two clinical trials, one with postpartum people and another with healthy adolescents. In this vaccine, a subunit of CMV, called glycoprotein B (gB), is combined with a novel adjuvant, MF59, a proprietary oil-in-water emulsion. Vaccinees were given either the gB/MF59 vaccine or placebo, then followed to assess any side effects experienced. Infants born to participants in one study were checked for CMV infection. Researchers sought to identify an association between those immune responses and the risk of CMV infection that could damage a fetal nervous system if the infection occurred during pregnancy. One aspect of the study that makes it unique is that it investigates the response of the most efficacious CMV vaccine tested at that time.
One hypothesis of this research was that antibodies in the blood of vaccinees should be correlated with vaccine efficacy. One challenge was that many antibodies generated in response to the vaccine being tested don't inhibit viral infection of cells, which is usually expected of antibodies that protect against a virus. Evidence has suggested non-neutralizing antibodies contribute to protection in other ways, but studies presenting that evidence lacked real statistical power. A key result of the study was the discovery that an antibody binding CMV gB on the surface of infected cells, but not free gB that's not associated with a cell, is associated with protection against CMV infection. This suggests that antibody binding to cell-associated gB is an immune correlate of vaccine efficacy.
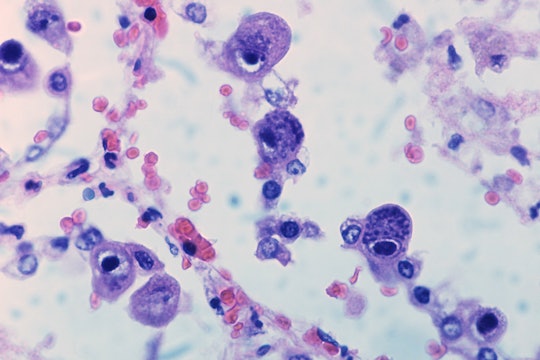
Pneumocytes (a type of cell in the lungs) infected with CMV. The central cell displays the dramatically enlarged nuclei characteristic of CMV
Via Wikimedia
Results of the study are expected to advance the development of a CMV vaccine that could protect developing infants. A CMV vaccine would also spare transplant recipients the need for expensive, limited antiviral treatments, improving patient survival and procedure success rates.
An obvious next step would be further studies to investigate immune responses and immune correlates of other CMV vaccine antigens. Another step would be to investigate correlates of protection against secondary infection and re-activation since this study focused on primary infection. Studies to confirm that the antibody response described in this paper could serve as an endpoint for vaccine development and evaluation is a third obvious next step.
CMV infection is almost universal and generally mild. But it can have devastating effects when infection occurs during pregnancy. CMV vaccines are hard to develop in part because of participants must be followed for years to determine vaccine efficacy. Studies defining immune correlates of protection, such as this one, facilitate the evaluation of vaccine efficacy and thus accelerate vaccine development.
To scientists, this study is important because it includes results of phase 2 clinical trials of a CMV vaccine; work at the forefront of a research effort that has been a focus of researchers across the world for decades. Also, the study results are expected to have a positive impact because they support understanding of human interaction with CMV. The CMV DNA genome of 236 kilobases (kb), encodes dozens of proteins, making it one of the largest and most complex viruses known to infect humans. By contrast, the sizes of the RNA genomes of influenza A, SARS-CoV-2, and HIV-1 are about 14 kb, 30 kb, and 10 kb. The size and complexity of CMV support many functions that allow CMV to sustain its lifelong, mostly-asymptomatic infection of humans. They also contribute to difficulty preventing and treating the infection in those who are vulnerable to serious effects.
This paper builds on previous research that shows the development of CMV vaccines can be helped by the identification of immune correlates of protection, signs in the immune response of vaccinees that show they're protected. It also builds on research describing the structure of the CMV virus and its interaction with the human immune response.
