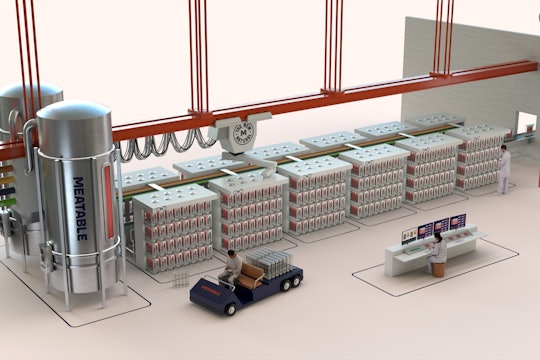
Why cultivated meat is about to have its Tesla Roadster moment
New cell-programming technology could help propel lab-grown meat into the mainstream
Almost everything we consider a great invention is, in fact, a series of great inventions. Take the electric car, which was dreamed up long before the gasoline engine, and had numerous rebirths and deaths throughout the 20th century, with a who’s who of great companies and inventors trying their hand at new prototypes. Unfortunately, its battery life remained too short and its top speed too slow to compete with the gas-guzzling competition. That is, until California engineer J.B. Straubel invented a new lithium-ion battery that solved both problems. His fateful meeting with Elon Musk in 2003 led to the creation of the Tesla Roadster — the beginning of the end for gasoline cars.
Cultivated meat — the industry I work in as a scientist — has followed a similar trajectory. In a 1931 essay, Winston Churchill already imagined growing meat in a lab without harming animals. This dream has also recently become a reality, driven by constant innovation and growing concerns about the carbon footprint of factory farms. Over the past ten years, I have witnessed it grow from a niche preoccupation into a global effort. But like the old electric car, cultivated meat has since been working through its prototype phase. What’s holding it back? The stem cells that are the driving force behind it run out of steam too quickly for efficient harvesting. The process of combining the necessary fat and muscle cells has proven too unwieldy. The resulting price tag is still too high. These hurdles — often dubbed our scalability problem — have prevented cultivated meat from making the transition from the lab to the market. Until now.
Today, seven years after Mark Post debuted the first lab-grown hamburger to the world, my company Meatable is unveiling a new invention that could give cultivated meat that final push from prototype to widely available product. Our new patented cell-programming technology, opti-ox, has already shown exceptional promise in overcoming our industry’s scalability problems. We are betting that this transition will eventually be looked back on as cultivated meat’s roadster moment — the crucial step that helps our industry get over the hump and into supermarkets, in pursuit of a more ecological world.
But how does our technology work? And what needs to happen next for it to fulfill its revolutionary potential? In what follows, I hope to offer some exclusive insights into this breakthrough and how we got there.
New Beginnings
Seven years ago, I was working on the research end of Mark Post’s pioneer hamburger project, and I remember feeling immensely proud. News cameras started flocking to our lab in the cardiovascular department of Maastricht University. Perhaps now my mother would stop asking me when I planned to get a real job. Mark’s famous patty showed the world that cultivated meat was a real and transformative possibility. To those of us working on the back end, the project also illustrated how far we still had to go for its prophecy to be fulfilled. Hundreds of thousands of dollars and tens of millions of cells (and the gargantuan lab space required for processing them) had gone into creating that burger. This was an early introduction to cultivated meat’s famous scalability problem.

10 layers of culture flasks used in the production of the first cultivated hamburger.
Photo by Daan Luining
In the following years, I was excited to work with start-ups around the world who devoted themselves to scaling up production while bringing down the price. The hope was huge. The success of our collective project could save countless animal lives and offer an ecological alternative to an industry with a deleterious carbon footprint. What needed to be done? I immersed myself in these questions while working as Research Director for Cellular Agriculture non-profit New Harvest, getting an overview of all the great work happening in cultivated meat and a crash course in what was holding us back.
The limitations were innate. Cultivated meat is made of cells, lots of them, ideally a combination of fat and muscle cells. Unfortunately for us, these mammalian cells are innately limited by something called “senescence.” Anatomist Leonard Hayflick discovered it in 1961: mammalian cells can only divide between forty to sixty times before they cannot divide any more. This limitation meant that cultivated meat makers had to keep harvesting and introducing new animal cells — a highly inefficient process.
We have long known of a possible way of overcoming this hurdle: using pluripotent cells. Discovered by Nobel Prize winner Shinya Yamanaka in 2006, these cells are special because they are able to divide endlessly, never running out of steam. The question was: how could we turn them into the specific cells we needed, muscle and fat? This requires shepherding the development of a cell all the way to adulthood. The available methods to do this have been time- and space-consuming. Until now.
Enter opti-ox
In the years after Mark Post’s famous hamburger, another Mark was (unwittingly) doing research that would prove crucial to the advancement of cultivated meat. Mark Kotter, a neurosurgeon and principal investigator at Cambridge’s STEM Cell Institute, spent years trying to reprogram pluripotent cells for neurological application.
Starting in 2014, Kotter discovered a way to build specific transcription factors into the pluripotent cell’s DNA. Every cell in the population turned into exactly what he wanted. He couldn’t believe his eyes. He had his graduate students re-run the experiment over and over. Every time, 100% of the pluripotent cells were converted to oligodendrocytes. He had created pure culture of cells from stem cells, which was unheard of thus far.
This turned out to have unintended applications, when I was introduced to Mark in 2016 through a New Harvest fellow. Mark had never heard of cultivated meat, but it took me maybe five minutes to convince him that it was exactly what the world needed. I realized just as quickly that opti-ox could be a game-changer for our industry. In 2018, we co-founded Meatable with our CEO Krijn de Nood, and started applying Mark’s technology to reprogramming pig cells. Soon, all our hard work started to pay off.
Humbling possibilities
In several experiments earlier this year, we have finally proved that we can grow pork using opti-ox technology on a porcine pluripotent cell. For the first time in history, we have successfully differentiated pluripotent cells into fat and muscle with unprecedented speed and efficiency.
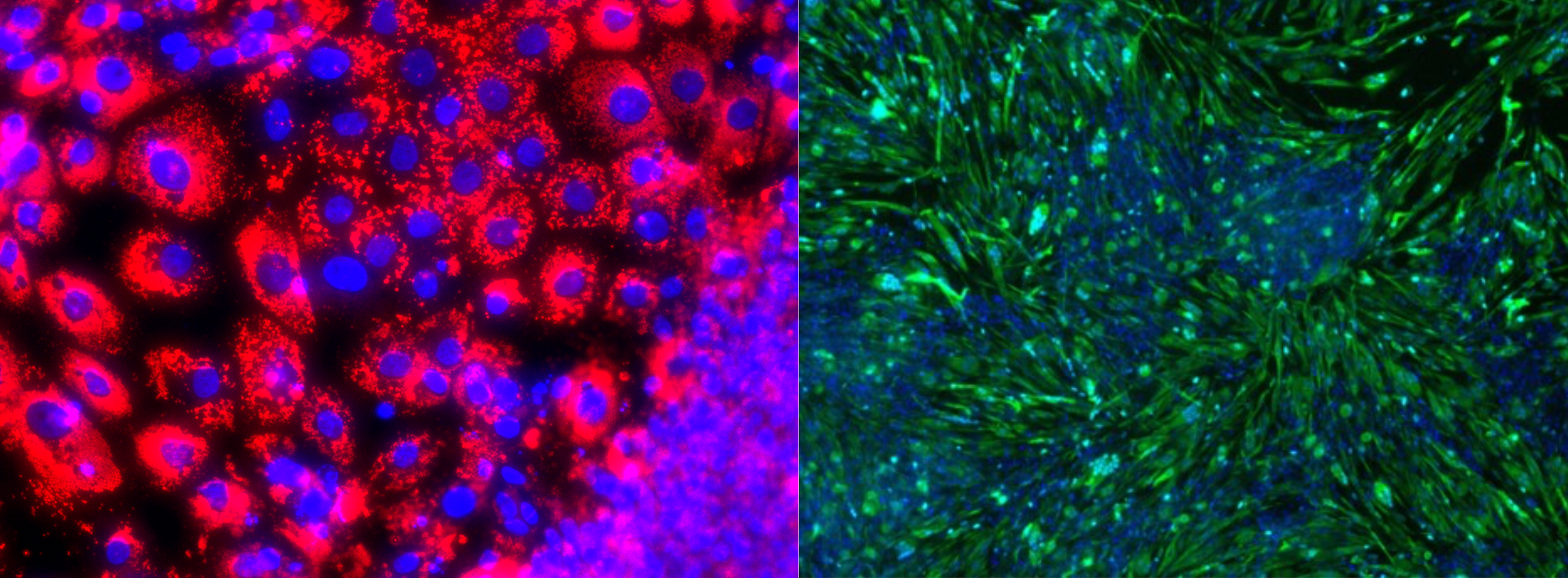
Pluripotent pork cells differentiated into fat (red) and muscle (green) in 7 days.
This process will allow us to grow fat and muscle together. An answer to an elusive problem: adult muscle and fat stem cells require vastly different — nearly opposite — environments to thrive. With pluripotent stem cells, muscle and fat cultures start from the same type of cell, so they can be grown side-by-side. No mixing required afterwards. The two ingredients can be grown together as they naturally do in animal meat. This, too, is a game changer. It will allow us to create a controlled large-scale process on both ends.
We believe that our opti-ox technology will be the battery pack that helps propel cultivated meat into the mainstream. Combining two breakthrough technologies that use cellular reprogramming in a way that has never been done before, the next test will be our first prototype sausage, followed by a pork chop. We then intend to continue our experiments with beef.
This technology will help us produce meat that's faster to market, higher quality, and more cost effective. More importantly, it will bring us one step closer to our greater goal: separating meat consumption from the death and carbon of factory farms, just as electric cars allowed us to drive without polluting the environment.
This reality may still be 5 years off, but in science that is little more than a blink of an eye. In the long-term, our success depends not only on scaling technological boundaries, but on challenging social perceptions, and having the courage to rethink one of humanity’s oldest habits. The possibilities are humbling.

