Inside the effort to create entire genomes from scratch
Yes, even human ones
Every living cell on our planet carries a genome: a collection of genes and other DNA that it inherits from its parents, who’ve inherited it from theirs, and so on, in a chain stretching back to the beginning of life itself.
And as life evolved from that primordial birth, genomes changed in infinitesimal increments, slowly becoming larger and more complex. They created new species and types of life-forms – minuscule bacteria; giant redwoods; and of course, all 7.4 billion of us humans, each with our own, slightly different genetic makeup.
Reading a genome is therefore incredibly informative. Though our genomes are nearly identical, the tiny fraction of a percentage that's unique can tell us where we come from, what diseases we might get, and what we traits and habits we may pass on to our children.
“What more powerful form of study of mankind could there be than to read our own instruction book?” asked Francis Collins, the current director of the National Institutes of Health, when scientists finally managed to sequence all the three billion DNA letters that make up the human genome in 2000.
How about writing it?
For the last decade, scientists from around the world have been trying to do just that by chemically stringing together the letters of DNA that make up a genome. The idea is that building genomes from scratch will answer fundamental questions about why our genomes look the way they do, and eventually, how we might change them. If genomes code for life, could we someday build an entirely man-made life-form? Or could we change our genomes so radically that we are no longer susceptible to viruses like HIV? These are the heady promises driving a new, rapidly advancing field of science called synthetic genomics.

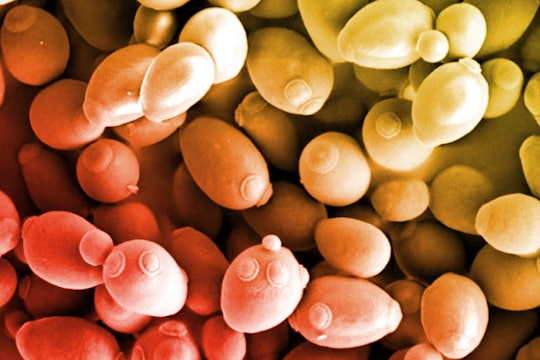
Single-celled brewer's yeast, imaged with a scanning electron microscope. Researchers are trying to re-create its genome from scratch.
Like Mother Nature before them, scientists first started writing the genomes of tiny bacteria and are now trying to make their way up the evolutionary tree to more advanced lifeforms. On this journey, a major milestone has always been Saccharomyces cerevisiae, the humble baker’s yeast. Though yeast is a single-celled organism with a relatively small genome, it's remarkably similar to ours. We share nearly 18 percent of genes (for comparison: bacteria share only about 1-5 percent). Also like our own genome, the yeast genome is partitioned into 16 separate pieces called chromosomes (we have 23).
Writing entire genomes from scratch – even for a single-celled organism like baker's yeast – seems like an absurdly challenging quest: DNA, inheritance, and genetics are more complicated than we originally imagined. One gene can take on many roles, and our genome is filled with ‘jumping genes,’ evolutionary remnants that make the landscape of the genome chaotic. But in a series of seven papers published just this year in Science, an international group of scientists called the Sc2.0 Project demonstrated that they could take large chunks of the yeast genome and replace them with manmade DNA.
That was step one. Next is to redesign life-forms rather than just copy them. Indeed, the Sc2.0 team is designing radical changes to the blueprint of natural yeast, rendering a new genome, called Sc2.0, which will be truly manmade. These changes were so extensive, and so risky, that lead scientist Jef Boeke, a geneticist at the NYU Langone Medical Center, admitted via email that at times he feared that the project might just “crash and burn.”
In a particular stroke of genius, Boeke’s team designed Sc2.0 so that if a specific protein is introduced into it, the entire genome breaks apart and shuffles back together to form a completely new genome. This means that once they’ve built one version of Sc2.0, scientists will be able to generate entirely new forms of yeast constantly, almost at the touch of a button. Their hope is that by controlling evolution at such a large scale, we’ll be able to generate new strains of yeast that could produce drugs like insulin faster and better. Sc2.0 is about halfway to completion.
Inspired by the successes of Sc2.0, a group of scientists (including many Sc2.0 members) announced last year that they wanted to start a large genome synthesis project called Genome Project-write, eventually aiming for a redesigned human genome.
This is a much more ambitious project. The genomes of multi-cellular organisms are more complex than that of yeast and might be much less tolerant of human-made design choices.
Creating humans is also an ethical minefield. Unsettled questions about who might own a synthetic human genome abound. Boeke warns that ownership could come down to who ends up funding the project development. Rob Carlson, a co-author of the GP-Write proposal, is even more skeptical of the idea of a patented artificial human genome, pointing out via email that “as soon as there is any possibility of a synthetic genome being used to germinate a live human, then ownership is obviously out of the question anyway…because you are now talking about owning a person.”
So far the GP-write project has been more talk than action, with large consultation meetings held between scientists and policy experts. The project has yet to attract significant funding. Perhaps successes in other organisms like yeast will embolden governments and private industry to open up to the idea of a man-made human genome.
Given that building a genome is so technically demanding, and with uncertain rewards, I’ve often wondered why anyone might want to undertake such a venture. Some, like Boeke, seem to be in it mainly for the science – for the questions that can only be answered by building life from scratch. Others, like Harvard geneticist George Church, a genome sequencing pioneer and co-author of the GP-write proposal, are more excited by the potential life-changing applications. In any case, it’s clear that writing genomes is the next major scientific frontier – and that we’re well on our way to crossing it.
