
Copyright Munch-museet/Munch Ellingsen Gruppen/Bono
Chemistry detectives figured out how to best preserve and display Edvard Munch's The Scream
New research suggests a better way to store deteriorating paintings
A sunset in Oslo; the clouds turn red in the sky. Edvard Munch is walking on a path on a fjord with some friends, and suddenly he seems to hear a scream. When he translates this onto canvas, it becomes the aptly named famous painting, The Scream.
There are four existing, original versions of The Scream. The 1910 version — the sole with tempera paint — is kept at the Munch Museum in Oslo. But if you are planning a visit to the museum, you will be disappointed: it is not viewable by tourists. Instead, it is kept in the dark to protect its colors. The yellow paint has faded to an off-white at some points in the sky and the neck of the central figure. It also shows signs of flaking (coming off in tiny pieces) in the lake region.
As old as art is, it took until the 20th century for art conservation to become an applied science, where we can use our chemistry knowledge to find out what causes art damage and how to prevent it. There are modern, non-invasive techniques available that we can apply to the art piece we are studying. These are demonstrated in a study published in Science Advances that revealed what is causing The Scream to flake and fade away.

Edvard Munch's The Scream
Copyright Munch-museet/Munch Ellingsen Gruppen/Bono
In the case of paintings, we can use X-rays to characterize the pigments, fabrics, and techniques an artist used. Infrared light can help unveil the original drawing and the paint loss. Further spectroscopical analysis can identify the varnish or layers of previous restorations that may have occurred over the past centuries. (These were often more damaging than helpful.) And once identified, chemists can choose the right solvent to remove them.
To help preserve Munch's masterpiece, researchers led by Costanza Miliani from the Italian National Research Council were called to understand why the yellow was fading and flaking. They were studying, in other words, the chemical composition of the colors. This was not an easy task, considering that Munch experimented extensively with synthetic pigments.
These synthetic pigments, more prone to fading or flaking than natural pigments, are known to cause visible aging in numerous modern art pieces, such as in many of Vincent van Gogh's paintings. The yellow is often based on cadmium sulfide (CdS), a yellow crystal that can exist in two different forms at the microscopic level, hexagonal and cubical.
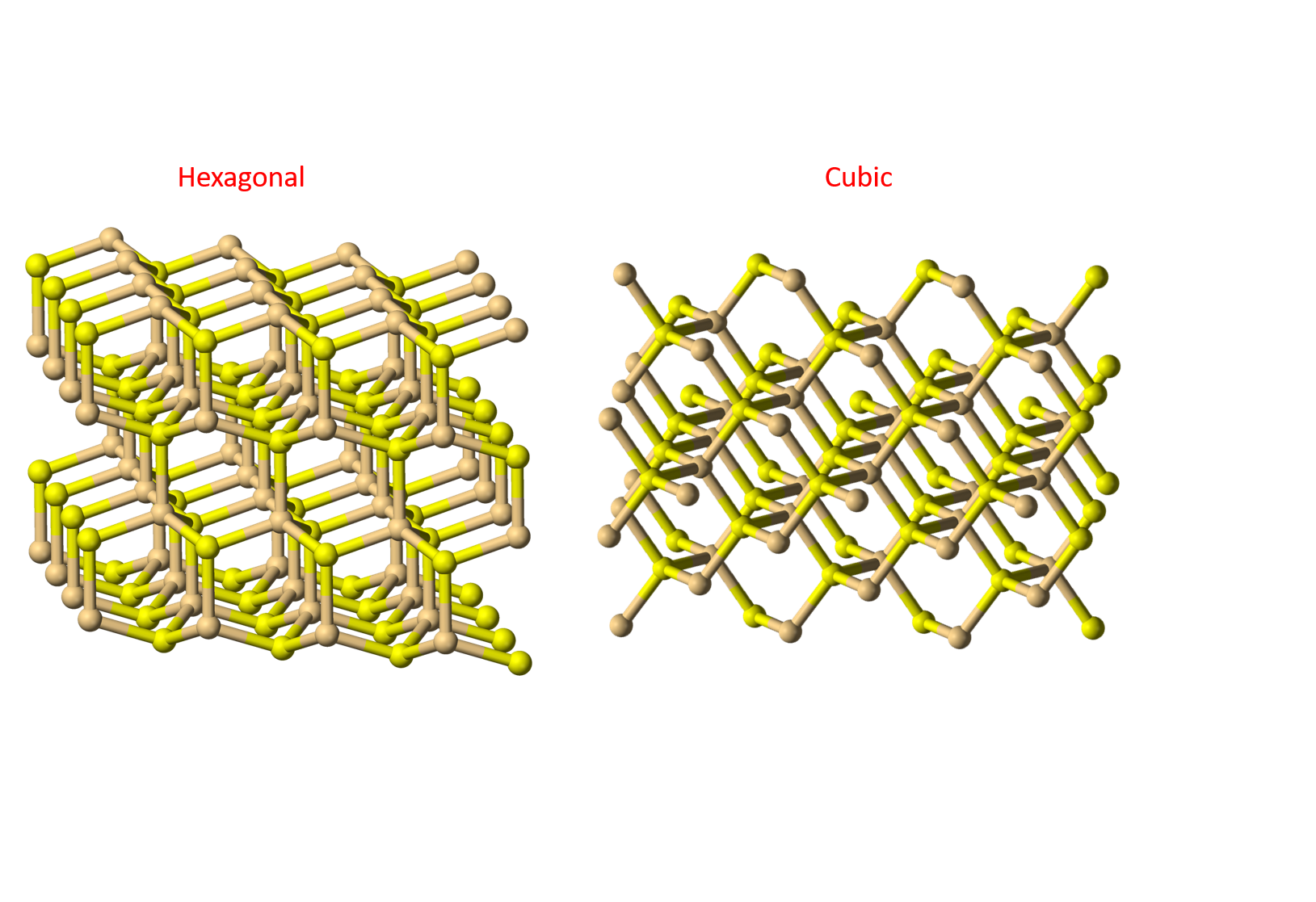
CdS can exist in two different forms at the microscopic level, hexagonal and cubical
Wikimedia CC 2.0
The manufacturing methods of CdS-based colors during the 19th and early 20th centuries left traces of other elements. For example, the so-called "wet process" of manufacturing paint would produce CdS often containing chlorine compounds, sulfites, and cadmium carbonates (CdCO3), making its yellow color more susceptible to fading. The researchers based their experiments on The Scream on previous studies that had found CdCO3 mixed with chlorine, sulfur, and sodium both in the discolored areas of the painting and where flaking was happening.
Is the presence of these extra elements related to pigment degradation? Which environmental factor plays a role in it? To answer these questions, the researchers carried out an initial series of experiments at the Munch Museum via the mobile laboratory (MOLAB): a set of portable equipment for non-destructive analyses of art pieces that cannot be moved for safety or fragility reasons. MOLAB offers a wide variety of techniques, with which they assessed the CdS in the paint, including its crystal structure (hexagonal or cubic) and the presence of other compounds.

The manufacturing methods of CdS-based colors during the 19th and early 20th centuries left traces of other elements
Henra / Pixabay
In the yellow sky and the lake water, Miliani and her colleagues found poorly crystalline hexagonal-CdS, CdCO3, and chlorinated compounds, signs that the wet process had been used. The researchers identified CdCO3 also where the yellow had faded, together with traces of hexagonal-CdS.
The wet manufacturing hypothesis was also supported by a more invasive analysis on the flaking area: six micro-samples (a thousandth of a millimeter square) were scraped off and analyzed at the European Synchrotron Radiation Facility in Grenoble, France. Notably, Miliani and her colleagues found sulfur compounds (sulfites and sulfates) probably formed as a result of CdS losing its electrons, a process called oxidation, over time.
To understand what environmental factors had contributed to the aging process, they prepared mock-up paints with an early 20th-century cadmium yellow powder – with properties similar to the lake area yellow on the original painting — and an actual oil paint used by Munch himself that the artist donated to the city of Oslo. The paints were artificially aged in different light and humidity conditions prior to analysis.
When comparing the paints with the mock-ups, they confirmed that they had additional sulfites and sulfates, a sign of CdS oxidation. This oxidation was caused by high humidity and the presence of cadmium chloride, and so the researchers were able to suggest a new way to store The Scream: in low humidity and normal light. These results could change the destiny of the painting, from being hidden in the dark to being displayed in a moisture-controlled room, accessible to the public once again.
"This work is very thorough, surely not easy considering the complexity of the materials on the surface of an artwork," said Rosangela Mastrangelo, a graduate student in Science for Conservation of Cultural Heritage at the University of Florence, who wasn't involved in the study. She continued, "Studies like these prove the importance of interdisciplinarity in the modern reality...In this process, chemists, conservator scientists, and restorers play a fundamental role in obtaining a good outcome. I believe that, despite some difficulties for the traditionalism of each discipline, this is becoming clearer. So much that, the EU is funding Science in Conservation of Cultural Heritage, not only to analyze but also to clean the art pieces and to implement innovative technologies to preserve them better."


Thank you for writing this article! It was a lot of fun reading it. I think that CNR researchers who found out the causes of color fading in Munch’s painting without taking large samples from it are depicting a new methodology in art conservation. Their work also describes many interesting details of art history. In the second half of the XIX century, color manufacturers for sure were not aware that chlorine and other impurities would have caused the color discoloration in paintings made by artists who bought their colors. It all began with color industrialization when John Rand invented in 1841 the first metal color tubes, and the first ready-to-use colors appeared in the market. Before, artists and painters were also craftsmen, who grind and mix colors with other substances on their own; they had a profound knowledge of the materials they were using, although they did not always guess as well. Afterward, this knowledge was almost lost, and most impressionists and painters relied on brand-new colors, for which they had little information about the duration. That’s why Van Gogh’s sunflowers turned from a brilliant to a dampened yellow, and the same happened to other impressionist paintings, like Edvard Munch as a matter of the fact. I strongly recommend you to read, if you haven’t already, Bright Earth by Philip Ball (there is also the Italian translation Colore, una biografia), which chronicles the story of color from ancient Egypt to nowadays.