
Robert-Owen-Wahl on Pixabay
New types of drugs, called PROTACs, melt cancer-causing proteins away
Researchers from Yale and Oregon Health and Science universities have now used this approach to treat chronic myeloid leukemia cells
What if you could melt the most dangerous parts of cancer cells into oblivion? Scientists are getting closer to making this a reality.
A team of chemical biologists from Yale University and Oregon Health and Science University, led by Yale postdoctoral researcher George Burslem, have shown that combining an existing cancer drug, imatinib, with a new form of drug that dramatically dissolves disease-causing proteins, can have powerful effects on chronic myeloid leukemia (CML) cancer cells.
Their findings, published recently in the journal Cancer Research, show that adding this therapy, called a PROTAC, to a standard cancer drug treatment can improve the effectiveness of the original drug.

Combination therapies containing more than one medicine can work better for some diseases than using single treatments
Photo by freestocks.org on Unsplash
CML is a type of cancer that affects bone marrow and the blood. It causes marrow to produce too many white blood cells, which can lead to tumor growth. It makes up about 15 percent of all leukemia cancers. According to the World Health Organization, around 100,000 people are affected by CML each year. In 2001, a breakthrough drug, imatinib, sometimes known by its commercial name Gleevec, was approved for treating CML patients.
Imatinib is seen as a landmark drug because it was the first of its kind to target a specific process in cancer cells that isn’t found in healthy cells (instead of just targeting rapidly dividing cells in general). In CML, the difference is caused by a mutated version of a protein called ABL1.
Proteins are molecular machines in our cells that carry out all sorts of jobs. Rogue ABL1 proteins work in combination with other proteins to command CML cells to replicate uncontrollably. Imatinib works by plugging a gap in the ABL1 protein, which stops it from carrying out its mission.
Although it was an important discovery in so-called "targeted cancer drug" development, the effectiveness of imatinib could be improved. Some 80 percent of CML patients end up on the drug for life or, if their cancer mutates, they develop drug resistance and imatinib no longer works for them. The Yale and Oregon researchers sought to address this issue of drug resistance in CML patients.
Enter PROTACs: rather than just plugging a protein like imatinib does, PROTAC drugs make use of an existing process in our bodies for recycling unwanted proteins. The PROTAC latches onto the offending protein and then calls over other proteins in the body, called ligases, which mark the unwanted molecule as trash. The cell's waste disposal system then obliterates it.
PROTAC stands for “proteolysis targeting chimera.” “Proteolysis” literally means “breaking down proteins,” and a chimera is a fire-breathing female monster from Greek mythology that has a lion’s head, a goat’s body, and a serpent’s tail. This technology has been in development for over 20 years, with the first-ever PROTAC study published in 2001.
Much like a chimera, a PROTAC is made up of three parts: the head, which fits into the disease-causing protein; the tail, which recruits the destroyer ligases; and a molecular chain that links the head and the tail. When developing PROTACs, chemists vary the head, tail, and linker chain length to find the perfect molecule that will degrade their protein of choice.
Initially, the researchers tried making a PROTAC that plugged into the same gap in ABL1 as imatinib, but it didn’t work particularly well. It didn’t fully degrade all of the ABL1 in the cancer cells.
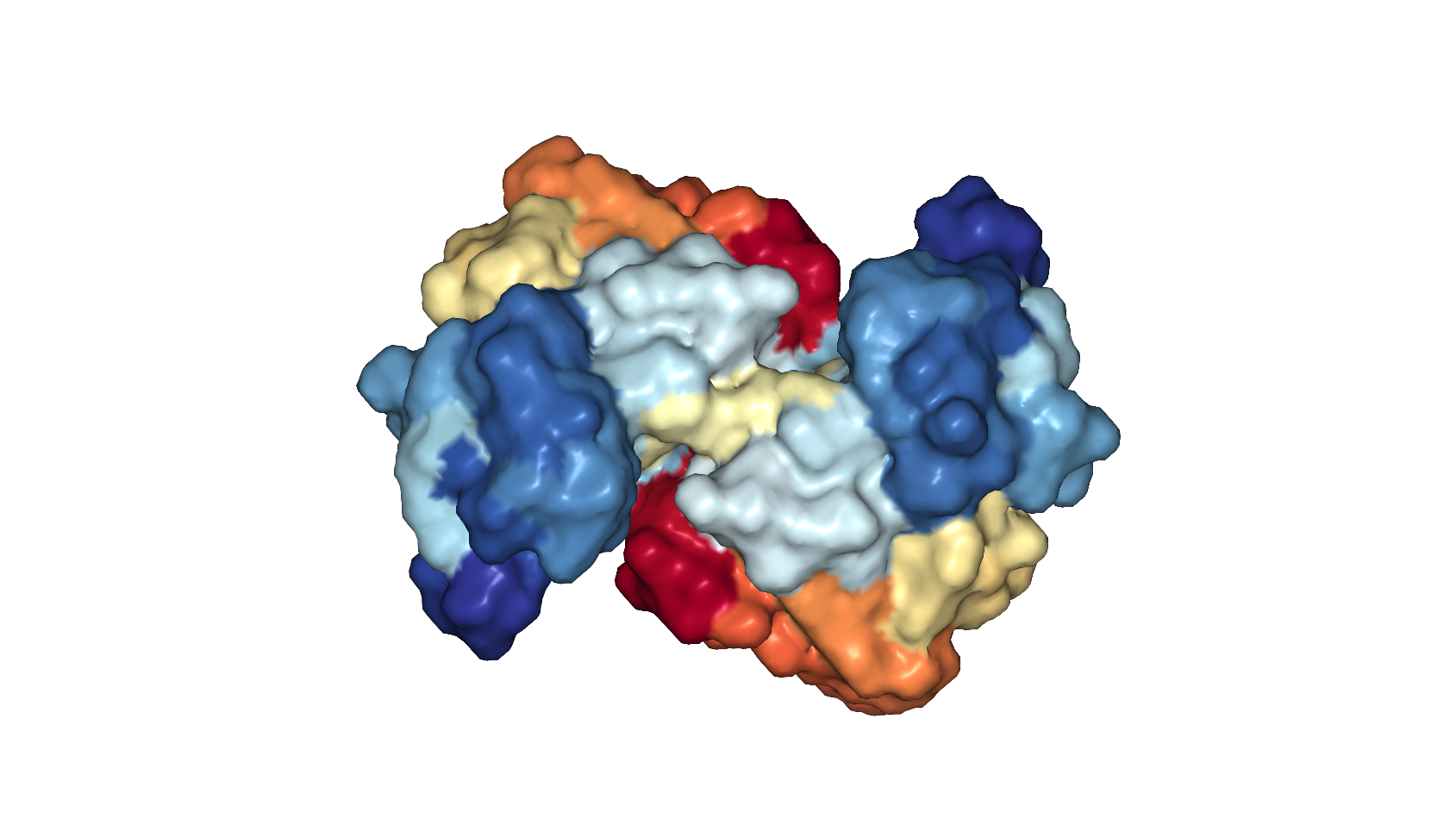
A surface view of the ABL1 protein, showing the pockets and crevices that a drug could target.
PDB: 6HD4. Schoepfer et al Journal of Medicinal Chemistry 2018
So they changed tactics and tried to find a pre-existing molecule that fits into a different pocket of ABL1 to incorporate into their PROTAC template. Proteins have large, complex, 3D shapes with all sorts of crevices, some better targets than others for designing molecules that fit into them. Sufficiently plugging a gap in a protein with a drug molecule stops the protein from being able to carry out its normal function, a bit like how getting a key (the drug in this case) stuck in a lock (the gap) prevents you from opening a door.
Starting with a known lab-tested molecule meant they could make the PROTACs much more quickly than if they were starting from scratch. Encouragingly, they were far more successful with this version. Burslem and his colleagues were able to show that treating CML cancer cells with a combination of imatinib and their newly developed PROTAC killed more cancer cells than imatinib alone could.

Plugging a protein stops it from functioning, much like what happens when you get a key stuck in a lock
They also showed this dual treatment didn’t have any effect on healthy cells. Combination therapies are becoming more common in cancer treatment to combat drug resistance, but this study is the first of its kind to combine a traditional drug with a PROTAC.
The discovery of this combination therapy gives a potential new option for CML patients who aren’t responding to imatinib by itself. Another PROTAC, designed to treat a form of drug-resistant prostate cancer is currently in clinical trials and, if successful, this ABL1 PROTAC is also likely to follow suit with animal and then, hopefully, patient trials.
There are now entire research conferences dedicated to PROTACs and this clinical trial will test whether the hype about this new kind of therapy is worthwhile. PROTAC research, in general, is becoming increasingly popular as researchers revisit proteins they previously thought were “undruggable,” such as the MYC (myelocytomatosis) oncogene, which can trigger multiple cancers, and tau, a protein involved in Alzheimer’s disease.
The rules of drug development are changing: proteins don’t just have to be plugged by small molecules these days. We can potentially apply old drugs in new ways using PROTAC technology. The combination of plugging and dissolving proteins could lead to another breakthrough in treating diseases like CML — a relief for tens of thousands of patients around the world.


I thought this was a nice overview and introduction to PROTACs, a very complicated topic to discuss with a lay audience! I would posit that the ending thought about “the rules of drug development are changing” is not right, though: PROTACs need to obey all the same rules as traditional drugs, namely, cell membrane penetrance, sufficient half-life, target specificity and selectivity. In fact, if PROTACs cause the rules of drug development to change in any way, it will be that they need to be more precise on the chemistry end since they now are coordinating a very intricate binary interaction between two proteins rather than simply blocking one from doing something. As the authors mention in the discussion, this could fail for any number of reasons such as the linker chemistry (length, rigidity, stability) or the type of ligase recruited (both VHL and CRBN are discussed in this paper). To combine all these thoughts, developing PROTAC-based drugs is more than twice as hard as developing a single protein binding drug based on coordinating the interaction of each component juuuuust right, but degrading rather than merely inhibiting a problematic protein can have vast advantages in putting cancer cells in check.