Scientists have figured out what a nasty gut bacterium eats. Can live biotherapeutics help?
They're the next big thing for treating C. diff infection, but developing them is a long process
Clostridioides difficile is not a welcome guest in the gut. Our gut microbiota — the community of helpful microbes that live peacefully in our intestine — protects us from colonization by this diarrhea-inducing bacterium. However, if these helpful gut microbes die, often as a result of antibiotic treatment, C. diff can grow with relative ease. When that happens, C. diff releases toxins that damage the lining of the intestine and trigger inflammation. In other words, C. diff moves in and sets the place on fire.
A recent study published in Nature Communications suggests that this inflammation liberates nutrients from host cells for C. diff to eat, while also suppressing growth of other, more helpful, bacteria that can consume those same nutrients. Ultimately, the pathogen's toxins "sort of create this niche that allows C. diff to persist in the gut environment at really high burdens,” says lead author on the study, Joshua Fletcher, a geneticist from North Carolina State University, now at University of Minnesota.
By getting a handle on the nutrients C. diff consumes in the gut, and what other bacteria can use those nutrients, Fletcher thinks we could design mixtures of bacteria (called live biotherapeutics) that interfere with C. diff's destructive lifestyle and treat infection.
Treating C. diff infection is tricky business. Antibiotics are currently the primary treatment option. But they are also a key risk factor for developing infection in the first place, due to their merciless destruction of the gut microbial community. While antibiotics resolve infection in many people, a subset falls into a vicious cycle of infection recurrence. These people take antibiotics to treat their initial infection but, because their gut microbes never recover, they develop C. diff infection symptoms again a few weeks later.
Some of these people receive fecal microbiota transplants (FMTs), in which they receive gut microbes from a healthy donor to restore resistance to the pathogen. FMTs “are extremely effective,” Fletcher says, however they are not standardized. “We still don’t know what all we are adding back, and in what amounts, and whether or not there are [other] pathogens.”
This has prompted scientists to develop live biotherapeutics that contain known and consistent mixtures of bacteria. Understanding how C. diff acquires food in the gut, what it eats, and the microbes that compete with it will help researchers design biotherapeutics that work like FMTs, but without the variability and risk. The idea of using live biotherapeutics to treat C. diff infection has gained interest in recent years. A number of studies have focused on identifying specific bacteria that can combat infection by reducing C. diff levels in the gut, including by forcing the pathogen to compete for nutrients.
In their study, Fletcher and his colleagues used C. diff-infected mice to show that toxin production promotes the expression of enzymes that break down the thick matrix surrounding intestinal cells. This dense mesh of collagen, and other proteins, fibers, and sugars, is rich in amino acids that C. diff likes to eat. Fletcher's team found that C. diff can grow by eating collagen or its degradation products. Based on these results, they think that the pathogen may capitalize on nutrients released from degradation of the extracellular matrix to survive and thrive during infection. The group also found that inflammation from C. diff toxins suppressed the growth of friendly gut bacteria that can eat these same nutrients.
Identifying bacteria that could "out-eat" C. diff may be a viable option for kicking the pathogen out of the gut. The question is, what does it take to turn intriguing findings from basic science studies like this into real, effective biotherapeutics for C. diff infection?
For one, we need microbes that can colonize the human gut. Many C. diff studies, like Fletcher's, use mouse models of disease, and mice do not have the same gut bacteria as humans. We need, “to understand the functional capabilities of those mouse bacteria and figure out which human bacteria can have the same effect,” says Taylor Feehley, Senior Director of Corporate Strategy and Development at a company that develops biotherapeutics for C. diff infection, among other diseases.
This is no easy task. Scientists must identify human bacterial strains with comparable curative properties as those observed in mice, a process that takes time and resources. And in the United States, the US Food and Drug Administration requires “robust characterization of the bacteria, whenever possible,” Feehley says. Screening bacteria for antibiotic resistance, for example, ensures that scientists are not promoting the spread of antibiotic-resistant microbes via their biotherapeutic.
Making a new drug requires more than just creating a promising mix of microbes. Researchers then have to figure out how they are going to manufacture and administer the live biotherapeutic to subjects in human trials.
Figuring out how to grow and deliver the bacteria to people may seem like a small logistical detail, but it is very important. Will the microbes be suspended in liquid and frozen? Will they be dried and packaged in a capsule? These considerations not only determine product efficacy and production strategy, but also ease of use (people are more likely to swallow a pill of bacteria than drink them).
If a drug candidate is lucky to make it past the process development hurdles, then comes clinical trials in humans. These can be costly. Even relatively small Phase 1 trials, which focus on product safety, run around $1-6 million.
Researchers must also decide who they will to test the product in, and what parameters they will measure to make sure the biotherapeutic works. "You can imagine measuring which bacteria get in [to the gut], how robustly they colonize, and how long they stick around," Feehley says.
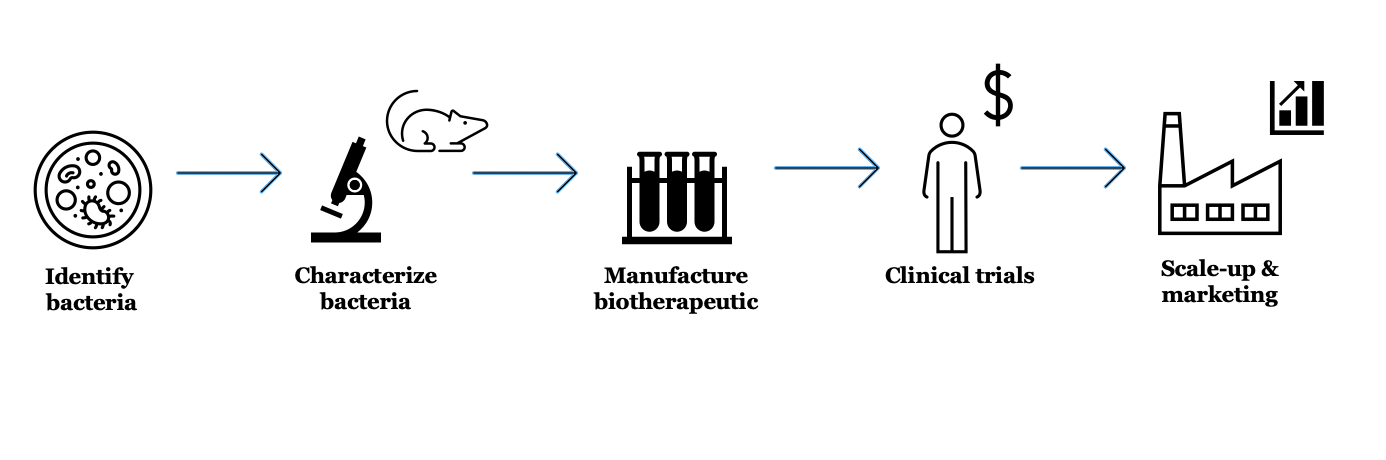
Developing biotherapeutics is a multi-step process– there is no guarantee a candidate drug will make it all the way through the pipeline
Madeline Barron
If a drug does not meet minimum thresholds for these factors, it may not make the cut. If the bacteria do survive in high amounts without negative side effects, then it is time to test if they actually do anything. Later trials involve determining how effective a biotherapeutic is, for example, at preventing recurrent C. diff infection in patients who have failed to get better using currently available therapies, suc as antibiotics or FMTs.
All of this takes time. Feehley says it generally takes about ten years to move from identifying promising microbial candidates in the lab to generating a biotherapeutic that is patient-ready. With that, Krishna Rao, a physician-scientist at University of Michigan, says that the most successful biotherapeutic candidates will target mechanisms “related or specific to C. diff's infection." This includes important aspects of the disease process that make C. diff so destructive, such as toxin production.
There are microbe-based therapeutics for C. diff infection at all stages in the clinical trial pipeline. Many candidates have roots in basic science studies, like that conducted by Fletcher's team. While Fletcher's work sits squarely in the conceptual realm of biotherapeutic possibilities, it could inform the development of something substantive down the line. Ultimately, by learning how C. diff survives and thrives in the gut, and the bacteria that get in the way, researchers are better positioned to develop biotherapeutics that survive the leap from lab bench to hospital bedside.




Great article! I like how the article flows gradually from basic science to clinical trials, while also highlighting important issues in manufacturing and production. At least from the source in the article, most clinical trials for live biotherapeutics seem to be in the US. I wonder if the composition of this “cocktail of bacteria” might have to be adapted for different populations across the world, considering different eating habits. As a vegan myself, I would personally be interested in how plant-based vs meat-based diets may also influence the treatment with such biotherapeutics.