
Harry Pot from Wikimedia Commons

Produced in partnership with TEDMED
Knowing more about how sneeze droplets spray can help prevent disease
Big and small droplets have different physics and even different pathogenic potential
In 1934, Williams Wells was the first scientist to convincingly describe airborne transmission of disease. He identified two main ways pathogens spread: large droplets, which fall due to gravity, and small droplets, which waft through the air as they evaporate. It is believed that pathogens like tuberculosis are transmitted through large droplets, whereas diseases like measles could through small ones, although evidence remain controversial and debated.
It may surprise you that for more than 80 years—despite new diseases, new means of travel, and new technology—our understanding of these basic routes haven’t changed much. Not until recently, when Lydia Bourouiba, associate professor at MIT and director of the Fluid Dynamics of Disease Transmission Laboratory, began to revisit these fundamentals and redefine how we think about respiratory disease transmission literally from the ground up.
Bourouiba began her career by studying the mathematics of how fluids flow, specifically looking at fluids with turbulent or chaotic flows. When she moved to Toronto shortly after the SARS epidemic, she realized that similar mathematical principles could be useful in modeling how diseases spread. That's when she began to use mathematics to improve epidemiology. “I started seeing these gaps in understanding transmission, and [seeing] that fluid dynamics could help fill such gaps,” explains Bourouiba.

Internet Archive Book Images via Flickr
Traditionally, scientists have created epidemiological models by developing equations, based on a variety of parameters that describe how diseases are transmitted between people and populations. However, many of these parameters are fitted to data and not based on physical principles — like how sneezing actually transmits disease, or what factors influence how far sneeze droplets may travel or persist.
Bourouiba thinks that improving the accuracy of these parameters can greatly improve the models and intervention strategies. “If one doesn’t have a mechanism to rationalize [the parameters] down to something we can directly measure, validate, and control, one ends up fitting data to models,” says Bourouiba, rather than designing models that incorporate underlaying physics. “One loses predictability power and ability to control.”
So Bourouiba moved to MIT as a postdoctoral fellow, and began to try to explain how diseases are transmitted globally based on how they are transmitted between you and your neighbor. Equipped with high-speed cameras, sophisticated models, and groups of patients, Bourouiba and her team are now answering fundamental questions about the mechanisms of disease transmission.
Many of these questions already have answers, like how long the ejection of sneezing lasts, where the large droplets land, or how small droplets can travel around a room. During TEDMED, Bourouiba showed how the physics of turbulent puff cloud of air emitted during exhalations, suspending and trapping drops within them, radically change the range of pathogen deposition and contamination. This shifts the paradigm away from the small versus large droplet framework of Wells into the mechanistic description of exhalations, including information of time and space, needed for monitoring, infection control and prevention, and risk assessments.
The next step is understanding how a sneeze can transmit viruses in the context of models that could impact the next year’s flu season. Her broad findings have already identified suggestions for disease control that can be implemented, influencing a variety of public health protocols and policies.
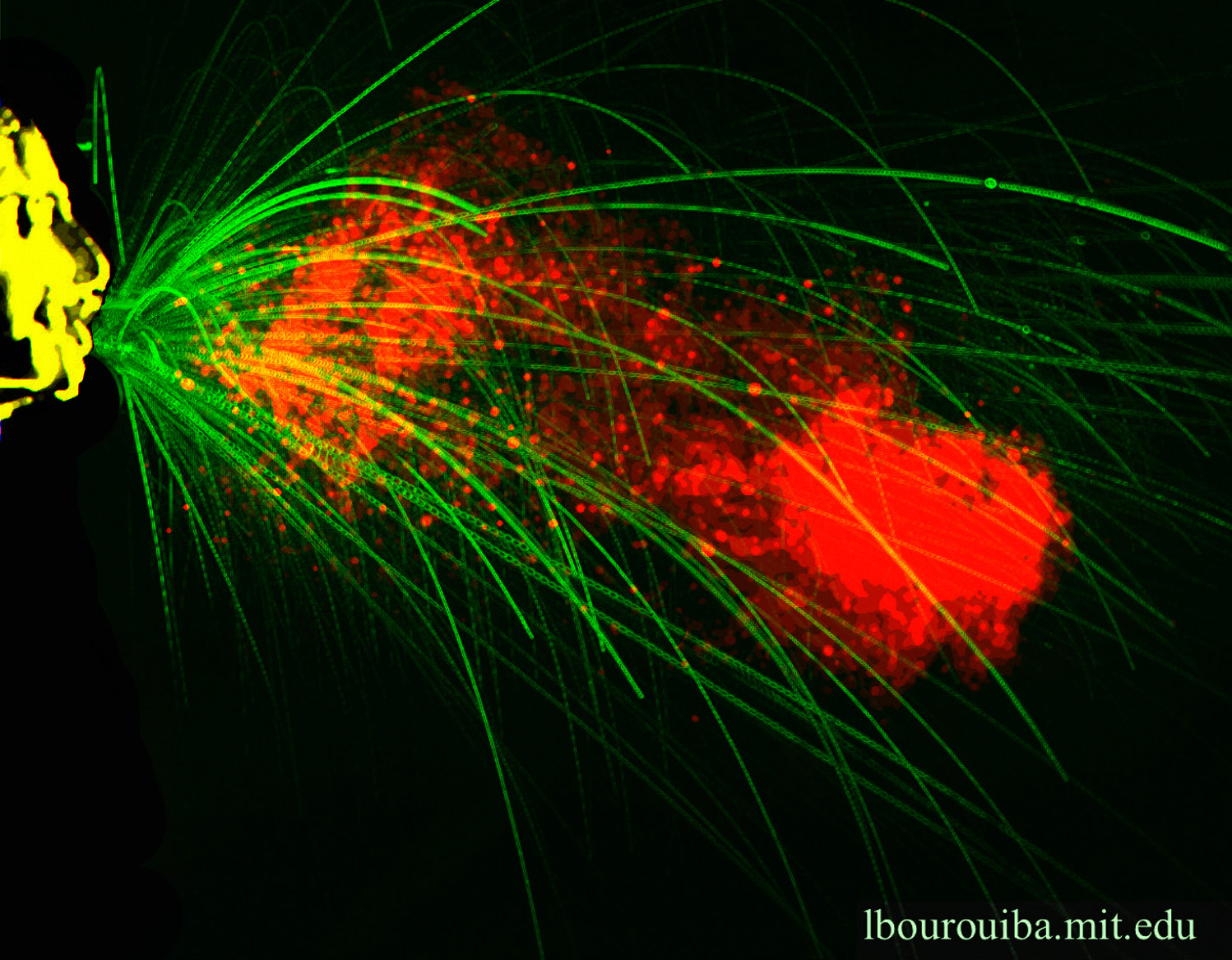
Lydia Bourouiba lab
But she still has further questions — like how the size of droplets can impact our susceptibility to disease. “The properties that exhalations and their payload influence the efficacy of infection upon exposure, for example influencing, their deposition in the lungs,” says Bourouiba. “We are working at elucidating the whole process, accounting for coupled physiology, immunology, microbiology, and fluid processes, to construct the full picture of those that have particularly high abilities to transmit certain respiratory diseases effectively. The properties that [the droplets] have initially can influence how far down they can get in the lungs,” says Bourouiba. “If we can understand the whole process, we could then determine — at the source — who would have better ability to transmit the disease effectively.”
This could inform how we manage numerous pathogens. Take tuberculosis, a disease that infects up to a third of the world’s population. Researchers know its symptoms begin deep in the lungs, but further characterizations of when, how, and why people produce infectious droplets could improve how we handle patient care and research.
Bourouiba is excited about a multi-year study she's working on in collaboration with clinicians, infection control specialists, microbiologists, immunologists, and virologists, for the study of transmission of influenza. Pioneering work in this interdisciplinary field isn’t easy. But Bourouiba says that ten to twenty years of this kind of research could lead to dramatic, tangible results, useful for a variety of pathogens. Considering the long and often uncertain process of developing new vaccines and diagnostics for infectious diseases, her approach to defining evidence-based prevention strategies is a vital piece of the puzzle. “You have to be doing both [prevention and treatment research]." It's also becoming ever more important. Because of rising antibiotic resistance, she explains, "We might be going into an era [similiar] to pre-antibiotic times, which is extremely concerning.”
Bourouiba's work is an important step toward redefining disease transmission, and infection control and prevention, moving the fundamentals from descriptions to measurable and quantifiable mechanisms. Truly understanding how people get each other sick will help us design protocols, policies, and tools to help people stay healthy and prevent epidemics and pandemics.
